Air Particulate Matter
In order to fully understand atmospheric processes and its impact on both the climate and human health detailed information on major components is necessary.
Air particulate matter (PM) is known for its adverse impact on health, as well as on continuously discussed climate changes. The building blocks of PM are metals, salts, organic species, and elemental carbon.
While inorganics are fairly well identified and quantified, only 20-50% of organics are usually characterized, mainly due to difficulties in their analysis. The organic fraction within PM can be differentiated into polar and non-polar species. The majority of the non-polar and mildly polar compounds have been found to be directly related to primary emissions (e.g., incomplete combustion of fossil fuels), and are quite well characterized using various organic solvent extraction techniques and GC-MS analysis.
Most of the more polar organic species are known to be formed through secondary processes (e.g., atmospheric oxidation) and have been found to partition more into the particle phase, forming secondary organic aerosols (SOA). Our laboratory focuses on evaluating the less polar organic species, focusing on determining the mechanism behind their formation through heterogeneous oxidation reactions and measuring their variability in the atmosphere.
PAHs and Their Oxidation Products
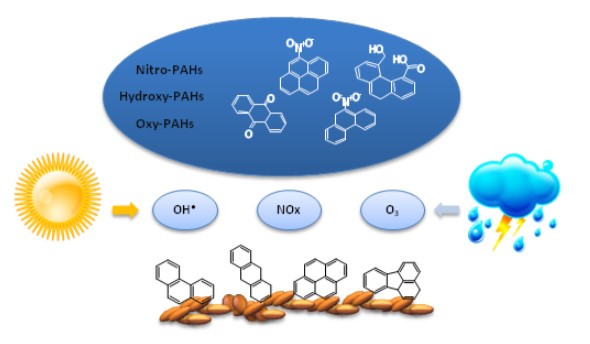
Polycyclic aromatic hydrocarbons (PAHs) are found in atmospheric aerosols, which are emitted to the atmosphere through incomplete combustion of fossil fuels. PAHs and their by-products are known to have carcinogenic and mutagenic activities. Both the kinetics of these reactions and pathways of their product formation are important in environmental studies.While in the atmosphere, PAHs partition between the gas and particle phases, with heavier species typically preferring the particle phase. Although many studies have determined the mechanisms behind their formation in the gas phase, only limited knowledge is available on the products of the heterogeneous reactions of PAHs adsorbed on particles.
Our research involves the development of a method for studying PAH reaction products in trace concentrations. This is followed by a study of the reactions of phenanthrene, anthracene, fluoranthene, and pyrene with reactive gas species focusing on the detailed identification of oxygenated products. These PAHs are highly representative due to partitioning into the particulate phase, so their high relative abundance is commonly observed in various forms of particulate matter.
Their reactions are simulated in a small-scale flow reactor with quartz filters used as the solid-phase support. Several oxidation products with multiple functional groups including aldehydes, carboxylic acids, ketones, hydroxy and nitro species were observed as a result of PAH reactions with gas-phase reactants such as NO2, O3, N2O5, and NO3. The identified reaction products help with further evaluation of product accumulation kinetics and their significance in atmospheric processes.
This work was funded by NSF Career grant ATM-0747349 and additional REU support.
Understanding Source Apportionment
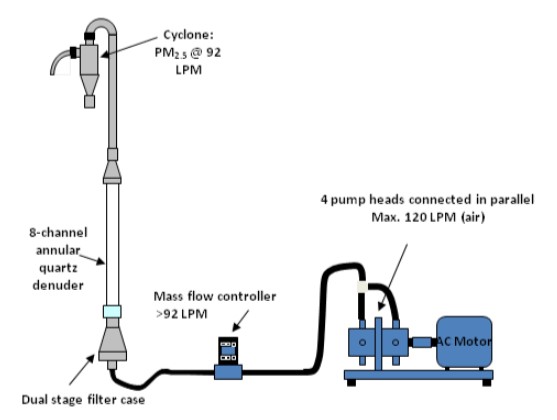
The currently used thermal desorption or extraction methods provide the characterization of only about ~30% of air particles (volatile/GC-elutable chemicals), thus leaving the remainder of organic carbon PM unaddressed. As a result, the PM health impacts or contribution to cloud condensation nucleation (i.e., rain) is poorly represented in atmospheric models. In our atmospheric chemistry research, we aim to accomplish mass balance closure – and then use it for detailed analysis. For this task, we use an organic/elemental carbon analyzer, which provides the overall concentration of organics in air particles. Its combination with TD-Pyr-GC-MS then enables detailed speciation.